- Startseite
- Forschung
- DFG-funded CCRC Graduate Program
- Cluster für Biologie des Leukozyten
- Cluster Wachstumsfaktoren des pulmonal-vaskulären Remodellings
- Immunzellantwort in kardiovaskulären Erkrankungen
- Inflammation im Rahmen der Herzinsuffizienz
- Strukturelle Herzerkrankung
- Klinische Kardiologie und Intensivmedizin
- Arbeitsgruppe Herzinsuffizienz
- Kardiale Zellersatz- & translationale Herzinsuffizienztherapie
- Elektrophysiologie
- Kontraktionskopplung und Calcium-Signaling
- Kardiovaskuläre Epidemiologie des Alterns
DFG-funded CCRC Graduate Program (GRK 2407)
Research Training Group
Inflammatory and cellular stress signaling: Switches to vascular dysfunction
Cologne Cardiovascular Research Center; University of Cologne

Spokesperson of RTG:
Prof. Stephan Baldus, MD (stephan.baldus@uk-koeln.de)
Deputy Spokesperson and scientific coordinator of RTG:
Univ.-Prof. Dr. Stephan Rosenkranz, MD (stephan.rosenkranz@uk-koeln.de)
Scientific Coordinator of RTG:
Monika Schlosser, PhD (ccrc-office@uk-koeln.de)
Kurzbeschreibung
Erkrankungen der Arterien wurden lange als Ausdruck strukturellen Umbaus durch das Zusammenspiel von Zellen, extrazellulärer Matrix und Cholesterinablagerung im Sinne der Atherosklerose gesehen. Neue Untersuchungen konnten zeigen, dass auch vaskuläre Stressoren, Signale auf Zellebene und inflammatorische Prozesse eine entscheidende Rolle bei Erkrankungen der Arterien spielen. Unsere Forschungsgruppe hat sich zum Ziel gesetzt diesen Ansatz im Rahmen der Grundlagenforschung zusammen mit Medizinern im „bench-to-bedside“-Ansatz diese Thematik weiter zu verfolgen und potentielle therapeutische Angriffspunkte so besser zu identifizieren.
Die Klinik für Kardiologie ist maßgeblich am Cologne Cardiovascular Research Center (CCRC) beteiligt, welches durch die Deutsche Forschungsgemeinschaft (DFG) gefördert wird, um junge Wissenschaftlerinnen und Wissenschaftler früh in der Karriere für dieses spannende und wichtige Feld zu gewinnen und auszubilden.
Short description
Arterial vascular disease has long been considered as a state of adverse structural remodeling within arteries upon accumulation of circulating and vessel wall-derived cells, matrix, and cholesterol – histological hallmarks of atherosclerosis. However, more subtle alterations of vascular homogeneity and in particular vascular stressors have emerged as critical pre-requisites and drivers for a large spectrum of diseases. The major aim of our collaborative research training group (RTG) is to identify common vascular stressors and overlapping or redundant cellular signaling responses across a broad range of vascular disease entities. Our RTG welcomes highly motivated PhD and MD students to actively pursue cutting-edge research on individual, yet coordinated research projects, that are directly related to answering pertaining key questions for specific diseases in this research field. This RTG, embedded into the Cologne Cardiovascular Research Center (CCRC) aims to educate the next generation of cardiovascular researchers and to foster better understanding of the translational needs in this important medical area.
Core research idea and main research focus
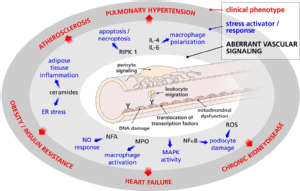
The overarching aim of this interdisciplinary and collaborative research initiative is to unravel common underlying mechanisms linking inflammatory stressors and cell signaling responses in arterial vascular disease. The identification of critical and redundant stressors as well as pertaining cellular signaling pathways that propagate dysintegrity of the arterial bed should be a prelude to revealing potential therapeutic targets for a wide range of different vascular diseases (Figure 3.1).
In this context, we will also consider mechanisms of inflammatory resolution. Since vascular dysfunction leads to a broad range of diseases and conditions, the expected gain of newly found knowledge and increased understanding should ultimately translate into the development of novel interventions, such as preventive paradigms, as well as new therapeutic options to treat vascular dysfunction in various organ systems and heart, lung and kidney in particular.
